GGRC – For Your Baby’s Health!
In modern medicine, screening programs hold a particularly important place in diagnostics and prevention. These programs facilitate the early detection of diseases, significantly increasing the chances of successful treatment. One such innovative system is ASTRAIA—a modern, multifunctional program specifically designed for women’s health screening and monitoring.
It is a prenatal screening program that integrates ultrasound examination, biochemical analysis, maternal personal data, and performs algorithmic evaluation based on the international standards of the Fetal Medicine Foundation (FMF).
ASTRAIA provides a higher level of accuracy and was first introduced in Georgia at the Georgian-German Reproductive Center (GGRC). Its purpose is the early assessment and prevention of fetal anomalies and pregnancy-related risks.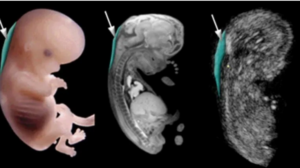
The history of ASTRAIA software began in the early 2000s, when there was a need for more precise, automated, and standardized management of prenatal screening procedures. The program was developed in Germany by a multidisciplinary team consisting of gynecologists, fetal medicine specialists, and software developers.
The main goal of the program was to reduce human error in risk assessment and diagnosis during prenatal screening and to establish a unified standard in clinical practice.
A significant milestone in ASTRAIA’s development was its collaboration with the Fetal Medicine Foundation (FMF) based in London, one of the world’s leading organizations in fetal medicine.
FMF’s established guidelines and statistical models were integrated into the ASTRAIA system, further enhancing its reliability and scientific value.
By the late 2000s, ASTRAIA was already widely used across Europe, especially in Germany, Austria, Switzerland, and the United Kingdom. It gradually expanded into Asia and then to North and South America.
Its multilingual support, localization, and adaptation to national healthcare standards have greatly contributed to its success. Each version of the software is continuously updated according to the latest guidelines.
Initially, ASTRAIA was available as a desktop version, operating only on a clinic’s internal network. Later, server-based and cloud-based versions were developed, enabling data sharing between institutions, automatic backup, and more flexible management.
Today, the program includes integration with ultrasound devices, laboratory information systems (LIS), and electronic medical records (EMR/EHR).
Dr. radiologist Lali Gogia, a certified of Astraia-at the Georgian-German Reproductive Center (GGRC), speaks about the importance of this new screening program:
“The first trimester of pregnancy (0–13 weeks) is a crucial period for embryonic development. During this stage, many genetic and structural anomalies can begin to manifest. That is why timely and high-quality screening is so important.
The ASTRAIA program assists clinical staff in the early diagnosis of genetic disorders, including Down syndrome (T21), Edwards syndrome (T18), and Patau syndrome (T13). ASTRAIA’s algorithms help determine whether a patient is in a high-risk group for preterm birth or preeclampsia.
It is also important that the specialist performing the early prenatal screening holds an international FMF certificate, which grants the authority to conduct diagnostics using the ASTRAIA platform. This certification must be renewed annually through a statistical audit of performed work and a certification exam, ensuring high diagnostic accuracy for risk assessment.”
What does ASTRAIA include?
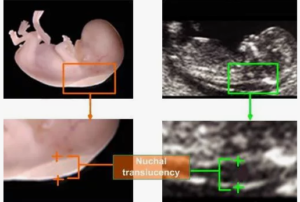
- Ultrasound examination between 11–13 weeks;
- Measurement of nuchal translucency and nasal bone assessment;
- Assessment of blood flow in uterine arteries;
- Blood tests (PAPP-A, free β-hCG);
- ASTRAIA software analysis for determining individual risk;
The ASTRAIA program evaluates not only the risks of preeclampsia and chromosomal abnormalities, but also provides information about:
- Intrauterine growth restriction (IUGR);
- Risk of preterm birth;
- Other potential complications that can be prevented through early diagnosis;
Who should undergo ASTRAIA screening?
First-trimester screening is recommended for all pregnant women, but it is especially important for the following groups:
- Women over the age of 35;
- Those with a family history of chromosomal disorders;
- Women undergoing in vitro fertilization (IVF);
- Women with chronic diseases (e.g., hypertension, diabetes);
- Women with a history of preeclampsia or fetal growth restriction;
Screening is performed only once during the pregnancy!
After the examination, it is essential to analyze the results, identify high-risk cases, and consult a geneticist if necessary.
Wishing you a healthy and successful pregnancy!
Leave a reply

how accessible is the ASTRAIA program for women in rural or underserved areas?
Reply